AMD gradually destroys a person’s sharp, central vision. It
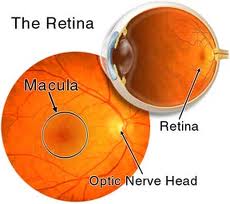
affects the macula, the part of the eye that allows people to see fine detail
needed to do daily tasks such as reading and driving.
There are two forms of AMD, a wet form and a dry form. The
wet form of AMD includes the growth of abnormal blood vessels. The blood
vessels can leak fluid into the central part of the retina, also known as the
macula. When fluid leaks into the macula, the macula thickens and vision loss
occurs. An early symptom of wet AMD occurs when straight lines appear to be
wavy.
“Eylea is an important new treatment option for adults with
wet AMD,” said Edward Cox, M.D., M.P.H, director of the Office of Antimicrobial
Products in FDA’s Center for Drug Evaluation and Research. “It is a potentially
blinding disease and the availability of new treatment options is important.”
The safety and effectiveness of Eylea was evaluated in two
clinical trials involving 2,412 adult patients. People in the study received
either Eylea or Lucentis (ranibizumab injection). The primary endpoint in each
study was a patient’s clearness of vision (visual acuity) after one year of
treatment.
Eylea is injected into the eye either every four weeks or
every eight weeks by an ophthalmologist. The studies showed that Eylea was as
effective as Lucentis in maintaining or improving visual acuity.
The most commonly reported side effects in patients
receiving Eylea included eye pain, blood at the injection site (conjunctival
hemorrhage), the appearance of floating spots in a person’s vision (vitreous
floaters), clouding of the eye lens (cataract), and an increase in eye
pressure.
Eylea should not be used in those who have an active eye
infection or active ocular inflammation. Eylea has not been studied in pregnant
women, so the treatment should be used only in pregnant women if the potential
benefits of the treatment outweigh any potential risks. Age related macular
degeneration does not occur in children and Eylea has not been studied in
children.
Other FDA-approved treatment options for wet AMD include:
Visudyne (verteporfin for injection) approved in 2000, Macugen (pegaptanib
sodium injection) approved in 2004, and Lucentis (ranibizumab injection)
approved in 2006.
Eylea is marketed by Tarrytown



5 comments:
This is superb,.i really like this share ,.it's too good,
."Cure for Macular Degeneration"
i was searching for some blogs about diseases cure,.
Thanks for sharing,
Liposomengel
It's good to see your post about medical,.
Mental illness
I was searching for something related to this type of illness,.
THanks for sharing.
medspa treatment
I have gone through an found your blog really helpful for me.
Charitable causes
Post a Comment